Multiphoton Intravital Microscopy
Principles and features of multiphoton microscopy
Multiphoton (MP) laser scanning microscopy uses pulsating light emitted from Titanium-sapphire (Ti:sa) lasers to combine photons and achieve the same excitation of fluorophores obtained by conventional single-photon laser-scanning microscopy. Compared with it, however, multiphoton microscopy carries features making it particularly useful for intravital imaging. Using excitation light of longer wavelength, which is less subject to scattering, MP microscopy can penetrate tissues more in-depth by several hundreds of µm. Further, its excitation happens only in a limited, dot-like, volume of sample, minimizing photo-toxicity and bleaching of fluorophores. Additionally, pulsating light from Ti:sa lasers allows using a non-linear optical process called harmonic generation (HG) to visualize unlabeled structures formed by molecules with specific conformations, such as collagen and myosin. Other unlabeled structures can also be detected based on the weak but identifiable fluorescence emitted by several metabolites, allowing sometimes mapping their relative abundance and spatial distribution.
Example of structures visible to MP microscopy without labelling: striated muscle imaged in living mouse. Blue = muscle A-band visible due to Harmonic generation (HG) signal from myosin. Yellow = organelles (I-band and perinuclear area) labeled by endogenous fluorescence of metabolites; different intensity of this signal between the three muscle fibers reflects their different metabolism.
Application to intravital imaging
Multiphoton microscopy can acquire images with a maximal 3D resolution of approximately 1 µm, thus only slightly less than the resolution achieved in single-photon confocal microscopy. Considering its other advantageous features, MP microscopy is therefore optimal for imaging living organisms with enough definition to recognize single cells and many subcellular structures. Its application to intravital imaging allows following over time microscopic details of many physio-pathological processes currently unreproducible using in-vitro models. Repeated longitudinal imaging of the same location is possible for weeks or even months, depending by the specific imaging model used; to this end, positional tracking methods help retrieving precisely the same site to observe through different imaging sessions. Compared to ex-vivo histological investigations of biopsies at different time points, longitudinal imaging of the same location carries the advantage of providing highly significant results using a smaller number of animals.
Longitudinal imaging of wound healing in bone: acquisitions of the same location inside a micro-lesion (400 microns-wide) in the parietal bone, respectively 1 and 5 days after the event. Image at day 5 shows invasion of cells (green) that will initiate the healing process. Bone tissue is visible in blue due to HG signal from collagen fibrils.
Requirements and limitations
Reaching a high level of image definition in-vivo comes together with some specific requirements and limitations, which must be carefully considered when deciding if this technique could suit your investigation.
For example, the considerable workload required for preparing and imaging each animal makes this method unsuitable for wide screening projects aiming at examining large numbers of animals. Also, the high magnification and resolution achieved entail a rather narrow maximal field of view (approximately between 0,5 x 0,5 and 1 x 1 mm, depending by the objective used); imaging larger areas is possible by performing overlapping acquisitions, but such procedure could be painfully time-consuming. Likewise, the sample to visualize must be positioned not more than few hundreds µm far from the objective; while this condition is easily satisfied when imaging external organs (e.g. skin), visualizing more in-depth location requires more complex solutions, such as installing imaging windows. Further and again due to the high magnification used, even the slightest movement of anesthetized animals can produce image artifacts; it is therefore essential to build specific tools able to keep very firm the part to visualize.
If your investigation requires high-resolution imaging of small tissue volumes in a convenient number of animals, MP microscopy can be a powerful tool for performing this task. Otherwise, if you aim at visualizing portion of tissues of several cubic mm in a larger number of animals (e.g. monitoring the size of tumors) you might consider other methods offering lower resolutions but greater image volumes, such as Magnetic Resonance Imaging, Computed Tomography and High-Frequency Ultrasound.
Intravital imaging models
Besides reproducing the physio-pathological conditions to be studied, imaging models adapted for intravital MP microscopy should also satisfy the additional requirements mentioned above. Further, and obviously, it is important that any type of cell or structure central to the investigation will be visible with this imaging technique. Due to its properties, MP microscopy can detect some unlabeled structures using harmonic generation or the fluorescence of endogenous cell metabolites. However, when aiming at detecting specific cell types or structures not offering this opportunity, fluorescent labelling of these must be implemented in the model. This is possible by using both genetic and chemical tools, such as transgenic mice expressing fluorescent proteins and/or injectable fluorescent tracers.
Use of imaging window in combination with fluorescent labelling of specific cell types in a transgenic mouse. MP intravital imaging of brain cortex in a mouse with fluorescently labelled cells (green = endothelial cells; red: other cell types).
Our service platform offers ready-to-go and well tested imaging models for visualizing different organs and tissues (e.g. skin, bone, brain cortex) and study various processes (e.g. cancer, wound-healing, stroke). When possible, we will develop new imaging models to enrich this repertoire and serve specific needs of research groups. However, building the necessary instruments and biological tools could require some time. For this reason, we recommend to first assess if any of our already established models can answer the questions posed by your investigation. If this is not the case, we will be happy to discuss the feasibility of a new model and provide an estimation of the time needed to establish and refine it.
Ex-vivo imaging
Besides in-vivo imaging of living anesthetized animals, MP microscopy is a powerful tool also for analyzing ex-vivo fixed or fresh tissue samples. Its enhanced in-depth imaging capability allows visualizing thick samples with intact organ architecture, eliminating cutting artifacts present in thin-sliced biopsies. We established methods for correlating in-vivo end ex-vivo imaging, which allow finding in biopsies the same locations previously visualized in living animals. Adequate permeabilization and clearing methods make possible to stain such biopsies, obtaining immunohistochemistry images that can be integrated with the dynamic data produced by intravital imaging.
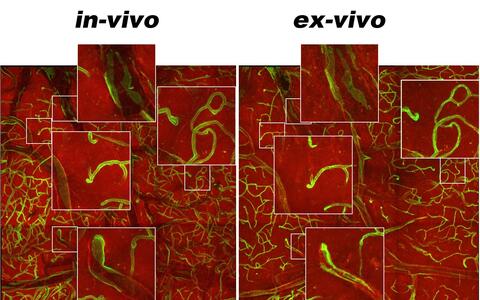
Correlation of in-vivo end ex-vivo imaging of brain cortex of a transgenic mouse expressing green and red fluorescent proteins in different cell types (green = endothelial cells; red: other cell types). Both images were obtained using MP microscopy, retrieving the same location previously imaged in-vivo in a whole-organ tissue biopsy. Magnified images show how thick tissue biopsies such this allow preserving tissue architecture in details often lost in thin-sliced biopsies used for normal confocal microscopy.
How we can help
Leica SP8 multiphoton microscope integrated with equipment for intravital imaging
We offer the service of a state-of-the-art Leica SP8 Dive multiphoton microscope integrated with the necessary equipment for imaging living mice and other small animals. The microscope features both two Ti:sa lasers for multiphoton excitation and tunable emission filters, which together allow simultaneous excitation and separate detection of signals from multiple fluorophores. A fluorescence lifetime imaging (FLIM) module completes this setup, allowing discerning even channels with similar color based on their different fluorescence lifetime.
Our collaboration with the MDC transgenics technology platform has been generating fluorescently labelled transgenic mouse strains specifically designed for this type of intravital imaging, and many others are available from external sources. We are also available to help with the generation of other tools (e.g. fluorescent reporters) and with the choice of any other material necessary for your experiments (e.g. contrasting agents).
This type of imaging poses problematics that can be properly solved only by carefully planning everything ahead and in details. We can assist in every step, starting from the design of the most appropriate imaging model and ending with the analysis of the data obtained from each microscopy session. Thus, if you think that multiphoton intravital microscopy could help your investigation, please contact us as earlier as possible and we will be happy to help you planning your experiment from the beginning.